Stabilization and crystallization mechanism of amorphous calcium carbonate
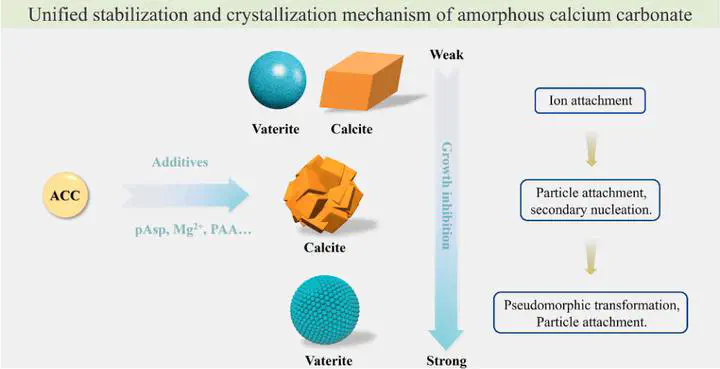
In February 2025, researchers led by Prof. Zhaoyong Zou from the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing at Wuhan University of Technology published a research article titled “Stabilization and crystallization mechanism of amorphous calcium carbonate” in the Journal of Colloid and Interface Science.
1.Research Background
Organisms often utilize amorphous phases as precursors to precisely fabricate high-performance biomaterials with delicate hierarchical structures. For example, amorphous calcium carbonate (ACC) transforms into calcite mesocrystals in sea urchin spines and aragonite platelets in the nacre of shells. These amorphous materials have attracted significant research interest due to their potential applications in catalysis and drug delivery. However, amorphous phases are typically thermodynamically unstable, making their stability and crystallization pathways difficult to control. Organisms employ various strategies, such as organic macromolecules, inorganic ions, and spatial confinement, to stabilize amorphous phases and regulate their crystallization processes. Therefore, understanding the mechanisms governing the stabilization and crystallization of amorphous materials is of great importance.
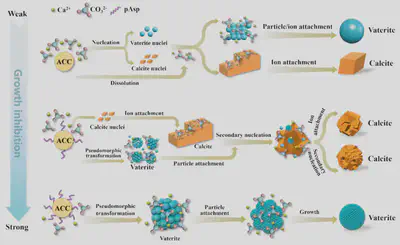
Calcium carbonate is one of the most common minerals in nature and the most abundant component of inorganic biominerals in invertebrates. Due to its simple chemical composition, calcium carbonate is widely used as a model system to study crystallization mechanisms. In solution, additive-free ACC typically crystallizes via a dissolution-recrystallization mechanism, transforming into a mixture of calcite and vaterite. The water content, water distribution, and particle size of ACC significantly influence its polymorph selection. In the presence of additives such as inorganic ions and polymers, ACC can also crystallize via solid-phase transformation, pseudomorphic transformation, and particle attachment mechanisms. Aspartic acid (Asp)-rich proteins are commonly involved in biomineralization, and poly(aspartic acid) (pAsp) is extensively used to study the crystallization pathways of calcium carbonate. Studies have shown that pAsp effectively stabilizes ACC and controls the polymorph selection and morphology of crystalline products. However, the influence of pAsp chain length on ACC crystallization pathways remains unclear.
2.Research Content
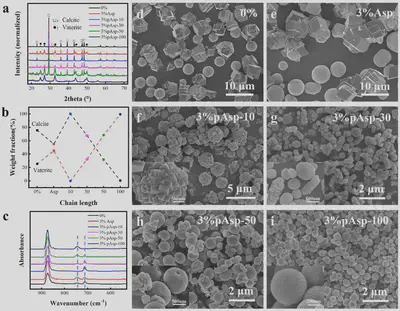
To address this, this work systematically investigated the effects of Asp monomer and pAsp with different chain lengths on the stability and crystallization pathways of ACC. The results revealed that the chain length and concentration of pAsp are key factors influencing ACC stability and crystallization mechanisms. By adjusting the chain length and concentration of pAsp, precise control over the polymorph selection and morphology of calcite and vaterite can be achieved. The chelating ability of pAsp with calcium ions increased with chain length, leading to an exponential increase in ACC stabilization time, while the Asp monomer had negligible effects on ACC stability. The Asp monomer promoted vaterite formation, whereas pAsp-10 resulted in pure calcite composed of aggregated small calcite crystals with a size of approximately 2–3 μm. As the pAsp chain length further increased, the calcite content decreased, and the vaterite content increased, with both calcite and vaterite crystals exhibiting reduced sizes.
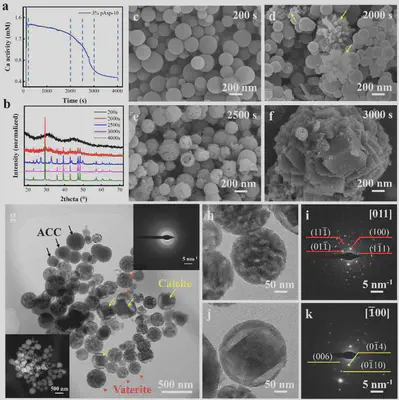
In the presence of 3% pAsp-10, ACC remained stable in solution for over 1800 s, maintaining its original morphology and particle size. At 2000 s, calcite nucleated first, but a significant amount of ACC nanoparticles remained. At 2500 s, vaterite appeared, and the surfaces of the nanoparticles became rough. TEM results indicated three distinct morphologies among the nanoparticles: the first type consisted of smooth, uniformly dense nanoparticles, typical of ACC; the second type showed nanoparticles with irregular contrast, identified as polycrystalline vaterite spheres with ring-like diffraction spots; the third type featured nanoparticles with a spherical shell and a central diamond-shaped crystal, identified as calcite. These results demonstrate that both calcite and vaterite can nucleate directly from ACC nanoparticles by stabilizing their surfaces.
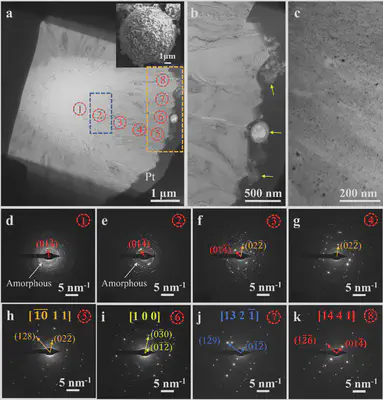
To further understand how pAsp influences calcite crystal growth, a thin slice was prepared from individual spherical calcite crystals using focused ion beam (FIB) technology. TEM images revealed nanoparticles attached to the calcite crystal surface and nano-sized pores ranging from a few to tens of nanometers. Electron diffraction patterns from different regions indicated that calcite crystallization began with the aggregation of amorphous nanoparticles, which gradually transformed into nanocrystals with different crystallographic orientations. Subsequently, calcite grew via nanoparticle attachment (possibly ACC, vaterite, or calcite). Due to relatively slow growth kinetics, crystals near the surface were more ordered than those in the interior.
3.Summary and Outlook
This study provides an in-depth exploration of the effects of pAsp with different chain lengths on the stability and crystallization pathways of ACC. It offers new insights into the stabilization and crystallization mechanisms in biomineralization and provides important guidelines for designing organic macromolecules to control the crystallization pathways, polymorphs, and morphologies of amorphous precursors. These findings enable scientists to better mimic natural mineralization processes, offering new strategies for materials science and biomaterial design.
This work was supported by the National Key Research and Development Program of China (2021YFA0715700), the National Natural Science Foundation of China (21905217 and 51832003), and the Hubei Provincial Innovation Group Project (2024AFA002).
Dr. Qihang Wang from Wuhan University of Technology is the first author of the article. Prof. Zhaoyong Zou and Associate Researcher Jingjing Xie from Wuhan University of Technology are the corresponding authors.
Publication Information: Qihang Wang, Wenyang Huang, Jilin Wang, Fei Long, Zhengyi Fu, Jingjing Xie* and Zhaoyong Zou*, Stabilization and crystallization mechanism of amorphous calcium carbonate. Journal of Colloid and Interface Science, 2025, 680: 24-35.