Nanocluster-Induced Liquid-like Precursor Formation and Crystallization: In Situ Visualization and 3D Reconstruction
 In situ visualization and 3D reconstruction of nanocluster-induced liquid precursor formation and crystallization
In situ visualization and 3D reconstruction of nanocluster-induced liquid precursor formation and crystallization
On March 6, 2025, Professor Zhaoyong Zou from Professor Zhengyi Fu’s team at the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, and Professor Guanbin Gao from Professor Taolei Sun’s team at the Hubei Key Laboratory of Nanomedicine for Neurodegenerative Diseases, published a research article titled “Nanocluster-Induced Liquid-like Precursor Formation and Crystallization: In Situ Visualization and 3D Reconstruction” in the Journal of the American Chemical Society.
1. Research Background
Biominerals are natural composite materials composed of organic and inorganic components, with their intricate structures closely associated with biological functions. It is widely acknowledged that biomacromolecules, particularly proteins and polysaccharides, play a crucial role in regulating the formation (including nucleation and growth) of inorganic minerals. Moreover, organic molecules can be incorporated into biogenic crystals, resulting in lattice distortions and enhancing the mechanical properties of biominerals. However, the precise mechanism underlying the guidance of inorganic mineral crystallization by organic molecules remains elusive, where a significant challenge stems from a limited comprehension of the dynamic migration process exhibited by organic molecules during mineralization.
During in vivo biomineralization, metastable amorphous phases such as amorphous calcium carbonate (ACC) and amorphous calcium phosphate (ACP) have been acknowledged as the key precursors of crystalline biominerals. Consequently, the influence of organic molecules on the formation, stability, and crystallization pathways of amorphous phases has garnered significant attention. One notable finding is that poly(carboxylic acid)s like poly(aspartic acid) (PAsp) and poly(acrylic acid) (PAA) can induce the formation of a transient “polymer-induced liquid precursor” (PILP) phase, which plays a pivotal role. However, current understanding primarily focuses on the impact of organic–inorganic interaction on the morphology and structure of inorganic crystals while lacking a comprehensive understanding of how organic matter actively participates in crystallization.
To address these issues, techniques like super-resolution fluorescence microscopy and electron tomography have been employed. However, fluorescent dyes exhibit limited influence on crystallization pathways and possess inherent limitations such as fluorescence quenching and functionalization challenges. Furthermore, additives like functionalized nanoparticles differ significantly from soluble macromolecules. Therefore, novel markers or innovative visualization techniques remain pivotal for direct observation of organic-molecule-induced inorganic mineralization processes. The team has long been dedicated to developing new material preparation technologies inspired by biological processes. Using synthetically produced inorganic nanoclusters to replace biomacromolecules can not only overcome the instability of biological molecules under some artificial synthesis conditions but also introduce functions and characteristics not possessed by biological molecules, thereby holding the potential to prepare functional materials with performance superior to natural biological materials.
2. Research Content
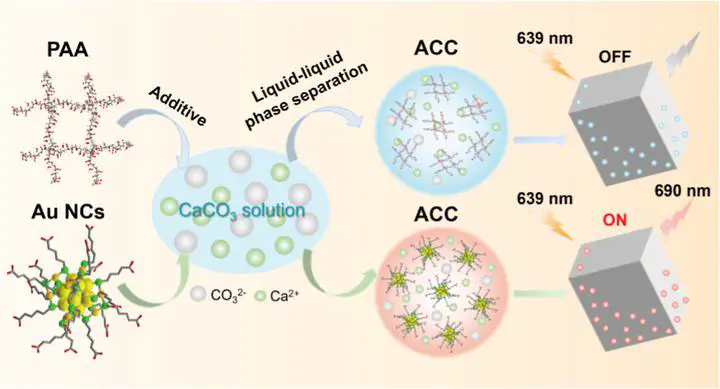
Recently, the team developed ultrasmall carboxyl-functionalized gold nanoclusters (Au NCs) with excellent photostability, non-quenched fluorescence, and a diameter of less than 3 nm as a substitute for polymers to investigate the organic-inorganic interactions during calcium carbonate crystallization, particularly the transient “Au NCs-induced liquid precursor” phase, analogous to the “polymer-induced liquid precursor” (PILP) (Figure 1a). Leveraging their higher density compared to polymers and stable inherent fluorescence, the formation and dynamics of Au NCs-containing liquid precursors were directly observed in real-time using light microscopy. The occlusion and 3D spatial distribution of Au NCs within calcium carbonate throughout the crystallization process were visualized through confocal fluorescence microscopy (CLSM) and transmission electron microscopy (TEM).
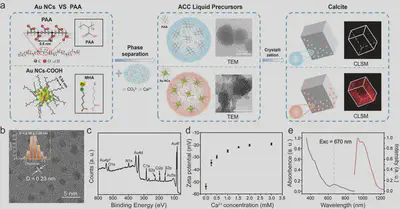
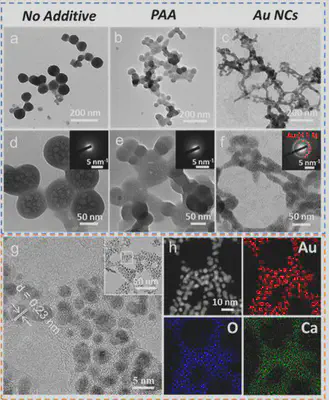
The study systematically analyzed the formation and characteristics of calcium carbonate precursors in the presence of PAA and Au NCs using TEM, SAED, STEM-DF, and EDX techniques. It was found that spherical amorphous nanoparticles formed in the absence of additives, while amorphous nanoparticles with liquid-like connectivity formed in the presence of PAA and Au NCs. Notably, unlike PAA, Au NCs were clearly visible, uniformly distributed within the calcium carbonate precursor, with minimal structural changes in the amorphous nanoparticles (Figure 2). These results indicate that acidic Au NCs can induce the formation of a calcium carbonate liquid precursor and serve as an alternative to acidic polymers.
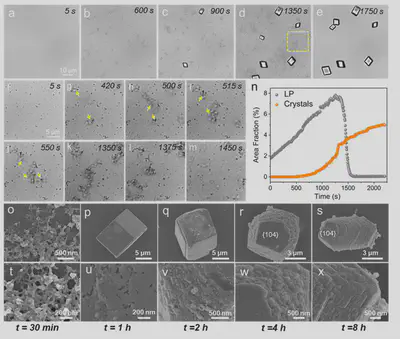
Utilizing the high density of Au NCs, the formation of the induced calcium carbonate liquid precursor and its crystallization process were observed in situ using optical microscopy. Upon introducing Ca²⁺, Au NCs-Ca²⁺ aggregates first formed. As ammonium carbonate diffused into the reaction solution, the CO₃²⁻ concentration gradually increased, generating numerous low-contrast liquid-like phases near the dense Au NCs-Ca²⁺ aggregates. Interestingly, these liquid-like phases often aggregated to form dynamic branched chains, and their dynamic association and dissociation processes were clearly observable (Figure 3). Around 500 seconds, small rhombohedral calcite crystals began to form in the solution. During this period, the densification of the liquid precursor intensified with dynamically adjusted morphology and size. It was observed that the liquid precursor attached to the crystal surface during growth. As crystal growth consumed Ca²⁺ and CO₃²⁻ ions, the depletion of free Ca²⁺ led to the consumption of Ca²⁺ from the liquid precursor, ultimately causing its dissociation and disappearance.
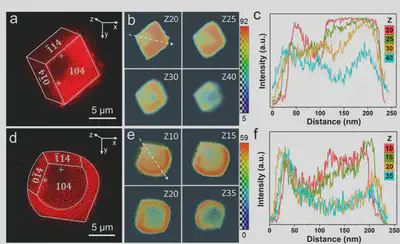
Based on the fluorescent properties of Au NCs, confocal microscopy observations revealed their selective adsorption behavior on specific calcite crystal surfaces. Z-axis analysis showed strong fluorescence at three corners on the crystal top (slice 20), with weaker signals in the obtuse corner region. As depth increased, fluorescence gradually enriched towards the acute corners. Near the bottom (slice 40), the fluorescence-deficient area shifted from the upper left to the lower right corner (Figure 4a-c). After 4 hours of growth, the crystal morphology changed significantly: the {104} faces exhibited rounded edges, while the {0$\overline{1}$<!-- -->{=html}4} and {1$\overline{1}$<!-- -->{=html}4} faces remained unchanged (Figure 4d). Fluorescence distribution showed Au NCs enriched at the rounded edges on the top surface, while the bottom surface displayed a completely opposite pattern (Figure 4e,f). The fluorescence intensity at the adsorbed regions on the rounded crystal edges was higher than in the surrounding areas, confirming that Au NCs adsorption was the key factor causing the morphological change of the {104} faces. This study successfully validated the potential of Au NCs as fluorescent probes for revealing polymer-crystal interface interaction mechanisms, providing a new method for visualizing crystallization processes.
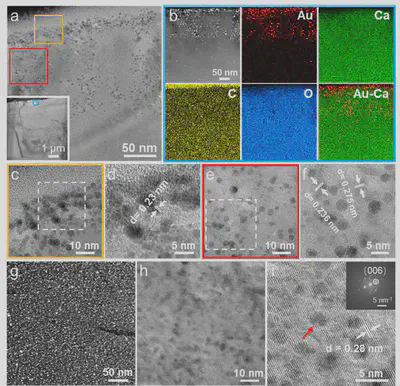
To elucidate the occlusion mechanism of Au NCs within calcite crystals, a 50 nm-thick calcite lamella was prepared using a focused ion beam (FIB) (Figure 5a). HAADF-EDX analysis showed homogeneous distribution of Ca, C, and O elements, but a higher Au concentration was found up to a depth of ~150 nm from the surface, with a Au/Ca atomic ratio of about 0.44. High-resolution imaging revealed that Au NCs existed as larger aggregates within an amorphous phase in the surface region, while beneath this region, they were more uniformly distributed within small crystalline domains of varying orientation (Figure 5b-f). This clearly demonstrates the involvement of the amorphous phase during calcite crystallization, facilitating the occlusion of Au NCs within the crystal lattice. In the central region, HRTEM showed lattice distortion around the Au NCs, disrupting the continuity of crystal lattice fringes, yet the corresponding FFT still exhibited single-crystal diffraction characteristics (Figure 5g-i). Notably, the Au/Ca atomic ratio at lattice defects was 5 times higher than in the central region, suggesting these defects might originate from stress generated during the crystallization of the amorphous phase. This study provides important evidence for understanding nanoparticle occlusion behavior in biomineralization.
3. Summary and Outlook
In this study, inspired by biomineralization processes, the team innovatively proposed using ultrasmall Au NCs functionalized with carboxyl groups and possessing stable fluorescence as substitutes for acidic polymers to induce CaCO₃ crystallization. The results clearly demonstrate the similarities between Au NCs and PAA and their effects on CaCO₃ crystallization. The dynamic aggregation process of the liquid precursor and the subsequent crystal crystallization process were effectively observed using optical microscopy and CLSM. Furthermore, the 3D spatial distribution of Au NCs within the crystals during CaCO₃ crystallization was directly observed using TEM, providing valuable insights into the occlusion mechanism of organic molecules in inorganic material crystallization. Overall, the team proposed a new strategy for studying material preparation and growth processes inspired by biological processes. Using functionalized Au NCs as analogs for acidic polymers not only allows a comprehensive understanding of organic-inorganic interactions during inorganic material crystallization but also provides a promising tool for in situ observation and multi-scale 3D visualization of crystallization mechanisms.
This work was supported by the National Key Research and Development Program of China (2021YFA0715700), the National Natural Science Foundation of China (52172287, 52273110), and the Hubei Provincial Natural Science Foundation of China (2024AFA002).
Jin Chen, a 2022 Ph.D. student at Wuhan University of Technology, and Professor Guanbin Gao are the co-first authors of the article. Professor Zhaoyong Zou, Professor Zhengyi Fu, and Professor Guanbin Gao from Wuhan University of Technology are the corresponding authors.
Publication Information: Jin Chen, Guanbin Gao, Zijun Zhang, Taolei Sun, Zhengyi Fu, and Zhaoyong Zou*, Nanocluster-Induced Liquid-like Precursor Formation and Crystallization: In Situ Visualization and 3D Reconstruction. Journal of the American Chemical Society, 2025, 147 (11): 9590-9600.